Moveo, a device for people with diabetes – Cross Between Modern Technology and Neurology
Source: eKapija
 Tuesday, 22.11.2022.
Tuesday, 22.11.2022.
 23:44
23:44
 Tuesday, 22.11.2022.
Tuesday, 22.11.2022.
 23:44
23:44
(Vladimir Jeftovic) Diabetic neuropathy is nowadays often detected late. One of the ways to in part regenerate the damaged nerve endings is specific physical activities. Divs Technology, led by the CEO Vladimir Jeftovic has developed an information and education device, Moveo, designed to show the condition of nerves in the peripheral nervous system.
As Jeftovic told eKapija back in 2021, the research-driven study is finished (pilot phase – phase 1 of clinical testing) and results were incredible.
– In 87% of cases, if a doctor confirmed the presence of neuropathy after using EMNG method (electromyoneurography), we confirmed neuropathy too. Even more relevant – in 95 % of cases if a doctor confirmed the absence of neuropathy, we would conclude the same using motion analysis.
As Jeftovic points out, it should be taken into consideration that an EMNG examination can last over two hours and is very painful, whereas the testing with Moveo device can be carried out at home in 10 minutes.
At the end of 2021, our project got the support of the Innovation Fund`s Mini Grants Program and that is when our progress accelerated, Jeftovic points out and adds that with the support of Plavi krug organization (Blue Circle, organization for people with diabetes), a survey was conducted on the potential technology and what diabetes patients thought about it and 98% of patients said that they would like to have that kind of technology for detection and monitoring of diabetic neuropathy.
The first test subjects that weren`t involved in the development of Moveo device could start using and testing the device as early as December.
– For the time being, Moveo isn`t an approved medical device and as such, can`t be used in standard medical practice for making decisions but we plan to get Moveo registered as a medical device after which it would be able to help doctors detect neuropathy quickly and easily, Jeftovic explains.
He adds that in the future, Moveo could be of use in the healthcare sector, facilitating screening procedures and most importantly – timely diagnosis. The device is very simple to use. There isn`t even an on/off button, instead, motion sensors turn on automatically when they detect the device is being held in the hand. Using Moveo requires no more than the basic functions on a mobile phone and no more complex activity than putting on gloves or socks.
– As for the application which performs and monitors testing , we devoted great effort to make it simple and intuitive to use and we will, of course, continue to improve the user experience in the next period, he says.
When it comes to movements the user performs and that are analyzed – they require little effort and strength, so older people wouldn`t have a problem performing them.
Moveo as an important tool for detection and prevention of neurological disorders
According to Jeftovic, an information engineer, Moveo technology isn`t only intended for diabetes. Diabetes is just one of many chronic conditions that cause nerve damage.
– Also, we believe that Moveo will be useful to people who just want to check and confirm they don`t have neurological problems. It could definitely be beneficial to people with prediabetes or those who are at high risk from diabetes, he states.
Jeftovic says that Moveo is an additional tool to monitor blood sugar levels and he adds that they believe this device will only further help the process and that they are planning to integrate their device with blood sugar level meters with the goal to give diabetes patients insight into how their motion score depends on their glucose monitoring.
Moveo to be available on the market in the second quarter of 2023
As Jeftovic emphasizes, the device is developed to the level of being used by end-users. However, the current obstacle to placing the product on the market is the production cost and capacity,
– We are actively engaged in lowering the production costs and we expect to place the producct on the local market in the second quarter of 2023, Jeftovic adds.
He further adds that the plan is for Moveo to get the status of a supplementary device first and eventually turn into a screening device. That would, naturally, require the transition from educational to diagnostic tool, which is no small feat, he states and adds that they are actively working on it.
– In the future, Moveo could potentially replace a part of the total number of EMNG examinations conducted or more importantly, provide specific early examinations for priority cases. For example, today a hospital usually has one electromyograph and one doctor can only conduct three exams per day if they only employ EMNG procedure. Plus, EMNG exams can be quite unpleasant for the patient, Jeftovic explains.
Research shows that over 80% of complications caused by diabetic neuropathy can be avoided if detected early and monitored properly.
– Our company`s greatest advantage is that it applies modern technologies and engineering approach to medical problems, Jeftovic adds.
According to him, they plan to broaden their scope of activity to general evaluation methodology of the nervous system, specifically peripheral nervous system.
– That entails conditions such as CIDP (Chronic inflammatory demyelinating polyneuropathy), CIPN (Chemotherapy-induced peripheral neuropathy) as well as recovering from a stroke. Then, biomarkers and validation technology for differential diagnosis of neurological disorders, concludes Jeftovic.
Divs Neuroinformatics was founded in 2019 with the idea to apply modern technologies to neurological sciences. The idea came naturally, as a link between two spheres – an IT engineer and a neurologist. At the end of 2020, when they adopted the shorter name for the company – Divs Technology, the idea emerged how to apply the knowledge on motion analysis to help patients with diabetes and how to promote regular health checks to wider population. Divs Technology mission is to help people prevent neurological
complications by providing available technologies to a wider population.
As Jeftovic told eKapija back in 2021, the research-driven study is finished (pilot phase – phase 1 of clinical testing) and results were incredible.
– In 87% of cases, if a doctor confirmed the presence of neuropathy after using EMNG method (electromyoneurography), we confirmed neuropathy too. Even more relevant – in 95 % of cases if a doctor confirmed the absence of neuropathy, we would conclude the same using motion analysis.
As Jeftovic points out, it should be taken into consideration that an EMNG examination can last over two hours and is very painful, whereas the testing with Moveo device can be carried out at home in 10 minutes.
Moveo device prototype (Photo: Eleonora Sergijević)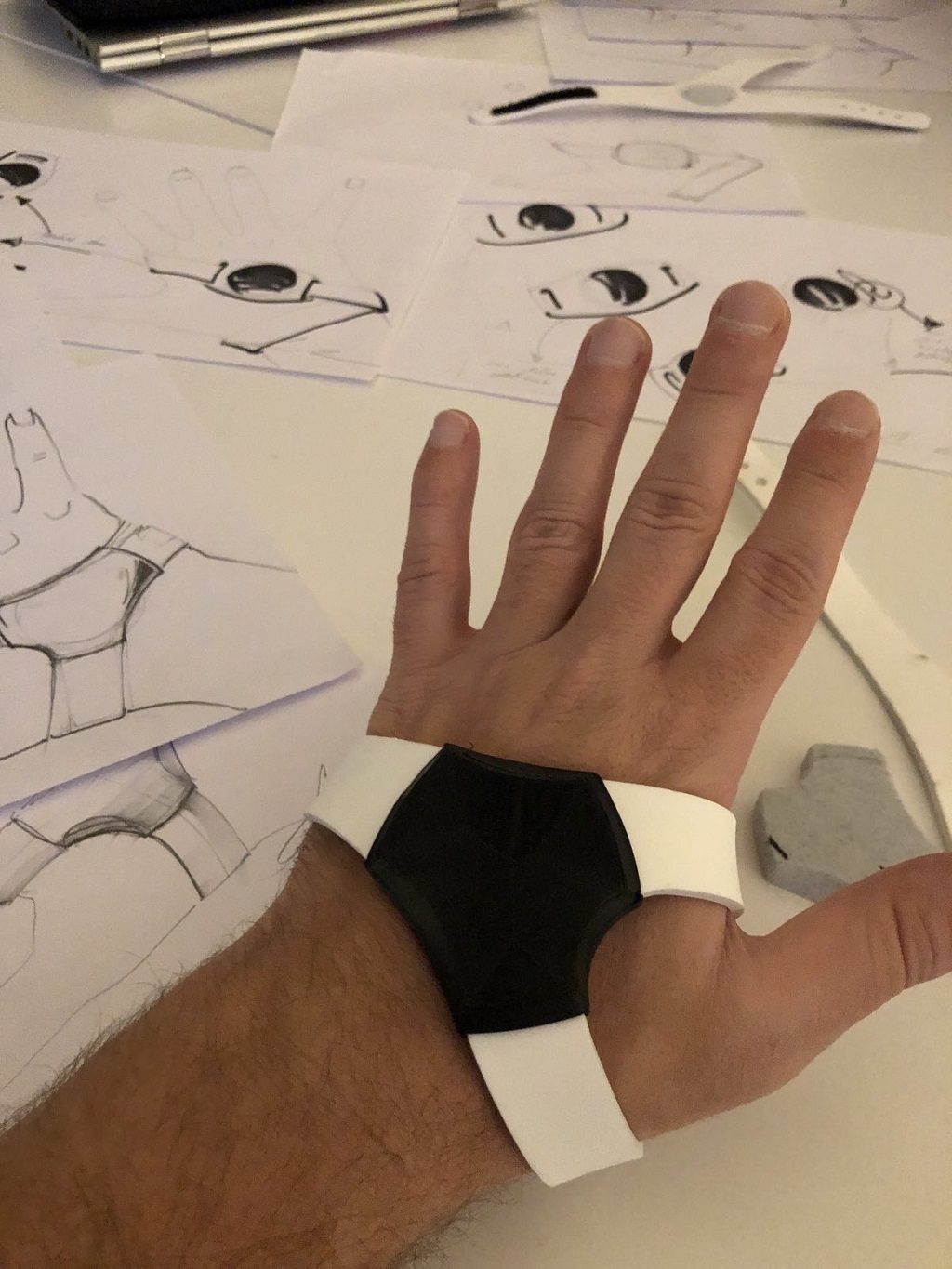

The first test subjects that weren`t involved in the development of Moveo device could start using and testing the device as early as December.
– For the time being, Moveo isn`t an approved medical device and as such, can`t be used in standard medical practice for making decisions but we plan to get Moveo registered as a medical device after which it would be able to help doctors detect neuropathy quickly and easily, Jeftovic explains.
He adds that in the future, Moveo could be of use in the healthcare sector, facilitating screening procedures and most importantly – timely diagnosis. The device is very simple to use. There isn`t even an on/off button, instead, motion sensors turn on automatically when they detect the device is being held in the hand. Using Moveo requires no more than the basic functions on a mobile phone and no more complex activity than putting on gloves or socks.
– As for the application which performs and monitors testing , we devoted great effort to make it simple and intuitive to use and we will, of course, continue to improve the user experience in the next period, he says.
When it comes to movements the user performs and that are analyzed – they require little effort and strength, so older people wouldn`t have a problem performing them.
Moveo as an important tool for detection and prevention of neurological disorders
According to Jeftovic, an information engineer, Moveo technology isn`t only intended for diabetes. Diabetes is just one of many chronic conditions that cause nerve damage.
– Also, we believe that Moveo will be useful to people who just want to check and confirm they don`t have neurological problems. It could definitely be beneficial to people with prediabetes or those who are at high risk from diabetes, he states.
Jeftovic says that Moveo is an additional tool to monitor blood sugar levels and he adds that they believe this device will only further help the process and that they are planning to integrate their device with blood sugar level meters with the goal to give diabetes patients insight into how their motion score depends on their glucose monitoring.
(Photo: Ivan Hristić)

Moveo to be available on the market in the second quarter of 2023
As Jeftovic emphasizes, the device is developed to the level of being used by end-users. However, the current obstacle to placing the product on the market is the production cost and capacity,
– We are actively engaged in lowering the production costs and we expect to place the producct on the local market in the second quarter of 2023, Jeftovic adds.
He further adds that the plan is for Moveo to get the status of a supplementary device first and eventually turn into a screening device. That would, naturally, require the transition from educational to diagnostic tool, which is no small feat, he states and adds that they are actively working on it.
– In the future, Moveo could potentially replace a part of the total number of EMNG examinations conducted or more importantly, provide specific early examinations for priority cases. For example, today a hospital usually has one electromyograph and one doctor can only conduct three exams per day if they only employ EMNG procedure. Plus, EMNG exams can be quite unpleasant for the patient, Jeftovic explains.
Research shows that over 80% of complications caused by diabetic neuropathy can be avoided if detected early and monitored properly.
– Our company`s greatest advantage is that it applies modern technologies and engineering approach to medical problems, Jeftovic adds.
According to him, they plan to broaden their scope of activity to general evaluation methodology of the nervous system, specifically peripheral nervous system.
– That entails conditions such as CIDP (Chronic inflammatory demyelinating polyneuropathy), CIPN (Chemotherapy-induced peripheral neuropathy) as well as recovering from a stroke. Then, biomarkers and validation technology for differential diagnosis of neurological disorders, concludes Jeftovic.
Companies:
 Fond za inovacionu delatnost Beograd
Fond za inovacionu delatnost Beograd
 Divs neuroinformatics doo Beograd
Divs neuroinformatics doo Beograd
 Udruženje za borbu protiv dijabetesa Plavi krug Beograd
Udruženje za borbu protiv dijabetesa Plavi krug Beograd
Tags:
Innovation Fund
Divs Technology
Plavi krug organization
Vladimir Jeftovic
Moveo
EMNG exam
state of peripheral nervous system
complications caused by diabetes
electromyoneurography
neuropathy research
testing Moveo devices
CIDP
Chronic inflammatory demyelinating polyneuropathy
CIPN
Chemotherapy induced peripheral neuropathy
special edition newsletter
Digital Transformation Roadmap to eFuture
Comments
Your comment
Most Important News
Full information is available only to commercial users-subscribers and it is necessary to log in.
Follow the news, tenders, grants, legal regulations and reports on our portal.
Registracija na eKapiji vam omogućava pristup potpunim informacijama i dnevnom biltenu
Naš dnevni ekonomski bilten će stizati na vašu mejl adresu krajem svakog radnog dana. Bilteni su personalizovani prema interesovanjima svakog korisnika zasebno,
uz konsultacije sa našim ekspertima.


 Izdanje Srbija
Izdanje Srbija Serbische Ausgabe
Serbische Ausgabe Izdanje BiH
Izdanje BiH Izdanje Crna Gora
Izdanje Crna Gora


 News
News











